Sodium hypochlorite has become widely used in both household and industrial settings for bleaching and disinfection. The origins of chlorine-based bleaching date back to 1774, when Swedish chemist Carl Wilhelm Scheele discovered chlorine and noted its bleaching properties in water. However, early applications in textile bleaching were unsuccessful due to fabric damage caused by the acidic by-products of chlorine reacting with water. In 1798, English chemist Charles Tennant advanced the field by producing calcium hypochlorite through the chlorination of lime slurry, offering a more practical and less damaging bleaching agent. He later patented a method for manufacturing bleaching powder by absorbing chlorine gas into dry lime hydrate, paving the way for safer and more effective chlorine-based disinfectants like sodium hypochlorite.
Sodium hypochlorite has become widely used in both household and industrial settings for bleaching and disinfection. The origins of chlorine-based bleaching date back to 1774, when Swedish chemist Carl Wilhelm Scheele discovered chlorine and noted its bleaching properties in water. However, early applications in textile bleaching were unsuccessful due to fabric damage caused by the acidic by-products of chlorine reacting with water. In 1798, English chemist Charles Tennant advanced the field by producing calcium hypochlorite through the chlorination of lime slurry, offering a more practical and less damaging bleaching agent. He later patented a method for manufacturing bleaching powder by absorbing chlorine gas into dry lime hydrate, paving the way for safer and more effective chlorine-based disinfectants like sodium hypochlorite.
AT A GLANCE:
Sodium hypochlorite remains a widely used and effective disinfectant due to its balance of practicality, stability, and antimicrobial efficacy.
Chlorine is highly soluble in water and reacts to form hypochlorous acid (HOCl), a powerful disinfectant. In alkaline conditions, HOCl dissociates into hypochlorite ions (OCl-), and all three species-chlorine, hypochlorous acid, and hypochlorite—exist in a dynamic equilibrium influenced by pH, as detailed below:
Cl2 + H2O HOCl OCl-
Increasing pH ->
The figure below shows the effect pH has on the equilibrium.
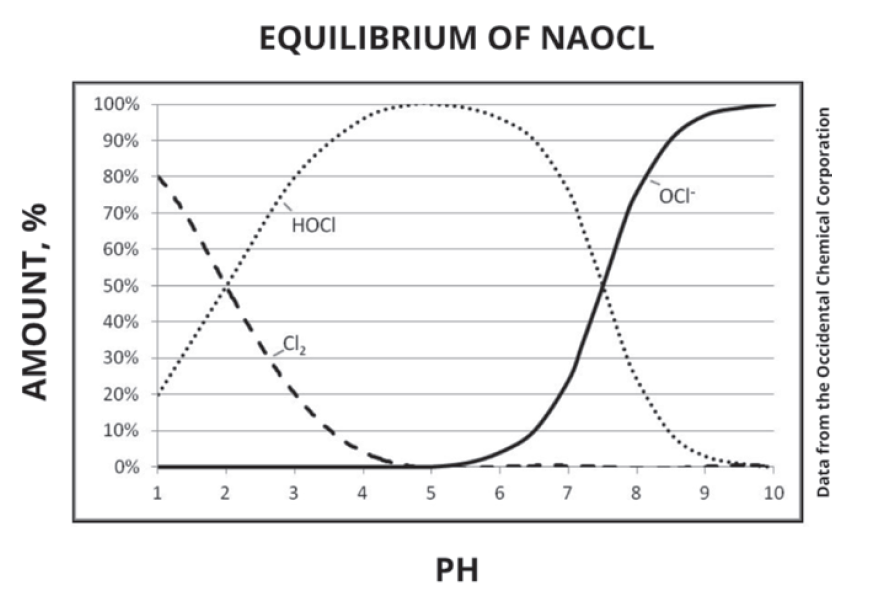
At low pH (below 2), chlorine dominates; between pH 2 and 7.4, HOCl is predominant; and above pH 7.4, hypochlorite becomes the main species. The most effective biological control occurs in the pH range of 5 to 7,
where HOCl is most abundant. However, HOCl is extremely unstable, making it difficult to handle directly. Instead, more stable hypochlorite salts are used, with sodium hypochlorite (NaOCl)—the active ingredient in household bleach—being the most common. Sodium hypochlorite offers a practical balance between stability and disinfectant efficacy, especially when managed under controlled conditions.
STABILITY
Sodium hypochlorite, while more stable than hypochlorous acid, is inherently unstable and begins decomposing immediately after preparation. However, with careful management, its decomposition rate can be minimized to produce relatively stable solutions. The stability and shelf life of sodium hypochlorite are influenced by five key factors: concentration, pH, temperature, presence of catalytic impurities, and exposure to light.
Lower concentration solutions decompose more slowly than higher ones; for example, a 15% solution decomposes about ten times faster than a 5% solution at 25°C. pH plays a critical role—solutions
are most stable between pH 12 and 13, where excess sodium hydroxide helps suppress decomposition, though excessive alkalinity can impair disinfecting action and damage materials. Temperature also affects stability, with higher temperatures accelerating breakdown; a 15% solution decomposes five times faster at 45°C than at 25°C. While cooler storage improves stability, freezing must be avoided. Impurities such as nickel, cobalt, copper, and iron catalyse decomposition and may cause discoloration.
Lastly, exposure to ultraviolet light speeds up degradation, so storing hypochlorite in opaque, sealed containers is essential to preserve its effectiveness.
EFFICACY
Sodium hypochlorite’s disinfectant efficacy is influenced by several interrelated factors. Its chemical activity depends heavily on pH, as it exists in solution as hypochlorous acid (HOCl) and hypochlorite ion (OCl-). HOCl is significantly more effective, and its concentration is highest between pH 6 and 7.5. At pH 9.5, although less HOCl is present, the solution remains moderately effective and more stable. The concentration of active chlorine also plays a key role—higher levels improve microbial kill rates but increase the chance of corrosion and toxicity. Over time, chlorine degrades, especially if exposed to light, heat, or air, which in
turn reduces efficacy. It is important to test products to ensure they are still efficacious at the end of shelf life, plus period after opening.
THE BALANCING ACT
The effectiveness of sodium hypochlorite as a disinfectant depends on the balance between pH, efficacy, and stability. In solution, it exists in equilibrium with hypochlorous acid (HOCl) and hypochlorite ion (OCl-), with pH determining which form dominates. HOCl is the most potent disinfectant, especially between pH 5 and 7, but it is highly unstable and difficult to manage. Sodium hypochlorite, a more stable salt of HOCl, is widely used instead due to its practicality and effectiveness, particularly in household bleach and industrial disinfectants. For stability, sodium hypochlorite solutions are best maintained at a high pH (12–13), where
decomposition is minimized. This stability comes at the cost of reduced efficacy, as the proportion of HOCl decreases with rising pH.
A compromise is often found around pH 9.5, where the solution retains disinfectant power while remaining relatively stable. Maintaining the right pH balance is essential – a lower pH enhances disinfectant efficacy but increases the risk of rapid degradation and corrosiveness, while a higher pH improves solution stability but reduces antimicrobial effectiveness. Therefore, controlling pH is essential to ensure sodium hypochlorite remains effective throughout its use and storage. At approximately pH 9.5, sodium hypochlorite solutions have been proven to be stable for around 18 months whilst still providing high levels of efficacy.
IN CONCLUSION
In conclusion, sodium hypochlorite remains a widely used and effective disinfectant due to its balance of practicality, stability, and antimicrobial efficacy. Its historical development and chemical behaviour highlight the importance of managing pH, concentration, and environmental conditions to optimise performance.
While hypochlorous acid is the most potent disinfecting species, its instability makes sodium hypochlorite the preferred alternative in both household and industrial applications.
Achieving the right balance between efficacy and stability—particularly around pH 9.5—ensures reliable disinfection while maintaining product shelf life and safety. Proper formulation, storage, and usage are essential to maximise the benefits of sodium hypochlorite and minimise degradation over time.